Generic Viagra market overheated

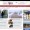
Generic versions of Viagra have flooded the domestic market following a ruling in May that American pharmaceutical firm Pfizer’s patented rights to use a chemical substance essential to treat erectile dysfunction had expired. Nearly 50 generic products by 28 companies have hit local shelves. / Korea Times file
The domestic pharmaceutical industry is struggling with the fallout of the American pharmaceutical firm Pfizer’s setback in the domestic anti-impotence drug market.
Nearly 50 kinds of Viagra generics from 28 companies have flooded the market following an Intellectual Property Tribunal’s ruling in May that Pfizer’s patented rights to use sildenafil, a chemical substance essential to treat erectile dysfunction, had expired. Pfizer claimed its sole use until 2014, but the tribunal declared the end of its exclusivity, citing patent terms.
Amid uphill competition, companies have made various forms of pills ㅡ chewable and powders ㅡ in order to meet the various demand from consumers. Yet the open competition has already created various side effects.
Several Viagra generic manufacturers have been caught running ads with exaggerated descriptions of their products, conducting over-the-counter sales, and bundling them with other medications as a marketing scheme, according to the Korea Food and Drug Administration (KFDA). Among newcomers to the fast growing market are CJ CheilJedang, Samjin Pharmaceutical, Ildong, Kolon, Daewoong and Dong Wha.
KFDA slapped a one-month sales ban on Hanmi Pharmaceutical for disclosing its product prices in commercial ads. Under the law, it is illegal for drug manufacturers and their wholesalers to disclose product prices through the media because it could cause excessive price competition. That ban, imposed on July 20, will continue until Aug. 19.
SK Chemical was slapped with a three-month sales ban for featuring a popular actress in its commercial targeting the general public. Under the law, generic Viagra is categorized as “special drug” whose advertisement is possible only through media outlets targeting doctors and pharmacists. The KFDA ruled SK’s contract with the actress was a strategy to promote the pill to the public.
A KFDA official said the government is trying hard to draw concrete guidelines for promotion of the drugs as soon as possible, adding more manufacturers are acting illegally in the face of overheated competition.
An insider warned that the situation has reached an alarming level, saying some companies promote their products as a cure-all.
“Some claim their generic drug can treat not only erectile dysfunction but also premature ejaculation, and prostatomegaly. But this claim is totally groundless,” a urologist said on condition of anonymity. “Many generic pill makers have hosted seminars for pharmacists and suggested handsome commissions in return. They even try to sell their products to doctors at departments that hardly ever prescribe anti-impotence drugs.”
The KFDA recently sent a letter to domestic generic manufacturers, calling on them to remain cautious until the release of the updated guidelines for their marketing and sales activities. In May, the authorities hosted a conference to explain to manufacturers about relevant rules and punishment against violators.
Industry insiders said the sales ban imposed on Hanmi and SK are viewed as the KFDA’s “warning” to market players.
“We are fully aware that the (generic drug) market is overheated,” an official said. “We will stay stern, merciless and astute in penalizing law-breaking companies.”
The medical circle is also gearing up self-regulation. The Korean Society for Sexual Medicine and Andrology (KSSMA) and an association for practicing urologists have launched a joint campaign to quench the steam and collect information regarding illegal marketing and sales activities.
“The two organizations have joined forces with the government to fight abusive use and consumption of the generic drug,” said Yang Sang-guk, a KSSMA executive. “We will do our best to protect people’s health.”
Korea’s erectile dysfunction drug market has continued to grow over the years. In 2007, the market was estimated at 77 billion won ($68.6 million). It grew to 97 billion won in 2010 and last year reached a 100 billion won, according to market statistics. The rise is expected to accelerate amid the growing elderly population. <The Korea Times/Park Si-soo>