
Vietnam begins Covid-19 vaccine trial on humans

By Lan Phong
Head of World News, Dantri Online Newspaper, Vietnam
HANOI: Vietnam on Thursday started the first trial Covid-19 vaccine shots on volunteers.
Three people were given the made-in-Vietnam Covid-19 vaccine at the Hanoi-based Military Medical Academy this morning, December 17.
The volunteers were provided with Nanocovax, a Covid-19 vaccine developed by Nanogen Pharmaceutical Biotechnology JSC.
Dr To Minh Sy, Nanogen Pharmaceutical Biotechnology JSC’s director for research and development, said that the volunteers have to stay at the immunisation room for at least 30 minutes for monitoring. They were instructed how to recognise possible reactions after the injection. The interval between the first and second injection will be 28 days.
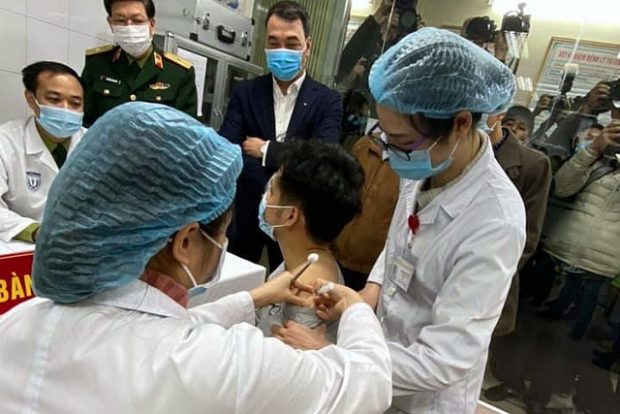
The participants will be closely monitored for 56 days and periodic follow-up evaluations will be followed out for six months from the first injection.
Based on the vaccine efficiency assessment of the first three during the 72 hours following vaccination, the researchers will determine the correct dosage for the remainder of the total 60 participants.
People who are selected for the vaccine trial must have good health and be aged between 18 and 50.
Lieutenant general, Do Quyet, director of the Military Medical Academy, said that safety is the first priority in the Covid-19 vaccine test. The academy set up 10 groups that are in charge of dealing with possible reactions after the vaccination.
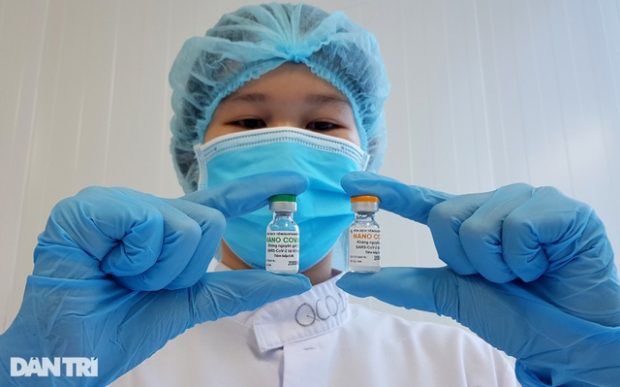
Earlier, in an interview with local media, Sy said that Nanocovax was produced following protein synthesis technology and repeated tests of toxicity and other tests on mice and monkeys have proved its safety and efficacy.
“There will be three clinical trial phases which are expected to complete by the fourth quarter of 2021 with the last phase seeing the participation of 30,000 people,” he said.
Sy also revealed that the expected price for a Nanocovax shot will be VND120,000 (USD5) and each person needs two shots.


